
The USP Expert Committee in Microbiology recently published a draft in-process revision of chapter <1223>, Validation of Alternative Microbiological Methods in the USP Pharmacopeial Forum (PF). Highlights include more detailed discussion and methods for validation parameters such as methods for demonstrating Limit of Detection, and multiple options and statistical calculations for... read more
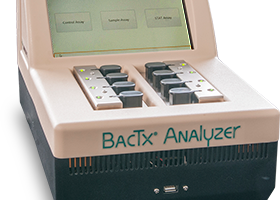
Researchers evaluating a rapid assay for detection of bacterial contamination in platelets suggested that a point-of-release assay using the BacTx (Immunetics, Inc.) might give an additional level of safety for platelet transfusion recipients. The BacTx assay is a colorimetric, qualitative, QC test for the detection of aerobic and anaerobic, Gram-positive and... read more
Australian researchers performed microbial recovery studies on Cord Blood Units (CBUs) by screening contaminated units after thaw, and also by performing spiking studies on fresh CBUs evaluating recovery both before freeze and again after thaw. These results were reported in the J. of Transfusion March 2014. The study demonstrated that a... read more



