Last year G-CON Manufacturing Inc. announced the launch of their 2nd generation POD® design for cellular therapy. The 2nd generation portfolio not only delivers design improvements compared to the current miniPOD CT, but it also represents a new POD® design. The CT line provides PODs® with unidirectional flow and corridor system within... read more

Should there be cause for additional concerns or worries in the cellular therapy laboratory (CTL) from the recent emergence of the Zika virus and its categorization as a relevant communicable disease agent or disease as defined in 21 CFR Part 1271? The FDA recently published the new guidance for HCT/Ps... read more

The USP Expert Committee in Microbiology recently published a draft in-process revision of chapter <1223>, Validation of Alternative Microbiological Methods in the USP Pharmacopeial Forum (PF). Highlights include more detailed discussion and methods for validation parameters such as methods for demonstrating Limit of Detection, and multiple options and statistical calculations for... read more
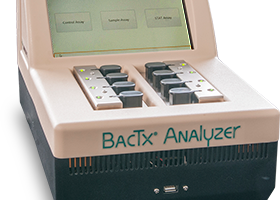
Researchers evaluating a rapid assay for detection of bacterial contamination in platelets suggested that a point-of-release assay using the BacTx (Immunetics, Inc.) might give an additional level of safety for platelet transfusion recipients. The BacTx assay is a colorimetric, qualitative, QC test for the detection of aerobic and anaerobic, Gram-positive and... read more
Australian researchers performed microbial recovery studies on Cord Blood Units (CBUs) by screening contaminated units after thaw, and also by performing spiking studies on fresh CBUs evaluating recovery both before freeze and again after thaw. These results were reported in the J. of Transfusion March 2014. The study demonstrated that a... read more

What is Cell Therapy Micro all about? First, as found in my mission statement, my primary focus is about protecting patient health by assisting cell therapy and regenerative medicine manufacturers in providing high quality and contamination-free products for their patients. Of course this is best accomplished through a variety of... read more






