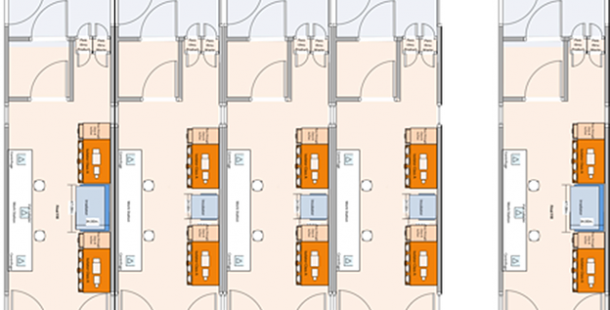
CT PODs: Prefab clean rooms for cell therapies
Last year G-CON Manufacturing Inc. announced the launch of their 2nd generation POD® design for cellular therapy. The 2nd generation portfolio not only delivers design improvements compared to the current miniPOD CT, but it also represents a new POD® design. The CT line provides PODs® with unidirectional flow and corridor system within 9’ and 12’ wide configurations. An example of the G-CON POD design and layout for cell therapy application can be found at the G-CON website here.
In my experience, the example design CT POD layouts with unidirectional flow are very similar to that of commercial manufacturing for the cell therapy product PROVENGE (Dendreon). Unidirectional flow having the clean room gowning entry and gowning exit as different rooms with one-way personnel flow on each end of the clean room core are best industry practice for aseptic manufacturing to help prevent contamination and cross-contamination. Furthermore, the material pass-throughs allow components and supplies to be transferred into the clean room separately rather than through the same area as the personnel.
For those considering expansion of cell therapy manufacturing capability or those wishing to bring cell therapy manufacturing in-house, the CT POD designs may represent a considerable reduction in cost of goods (COGS), or facility start-up costs.
